The special properties of platypus milk might hold the key to overcoming antibiotic resistance.
The duck-billed, web-footed, beaver-tailed, egg-laying platypus is often described as “God’s joke”, but the joke may be on antimicrobial resistant bacteria after the discovery of a unique protein in platypus milk.
Deakin University’s Dr Julie Sharp, a cellular and molecular biologist specialising in mammary gland biology and bioactive compounds in milk, has been investigating the special properties of wallaby, echidna and platypus milk for many years and the implications those properties could have for pre-term babies, in particular.
In 2010, Dr Sharp and her team at Deakin’s Institute for Frontier Materials (IFM) discovered an unidentified protein with antibacterial properties in platypus milk, and new findings this year have helped explain why those properties are so potent.
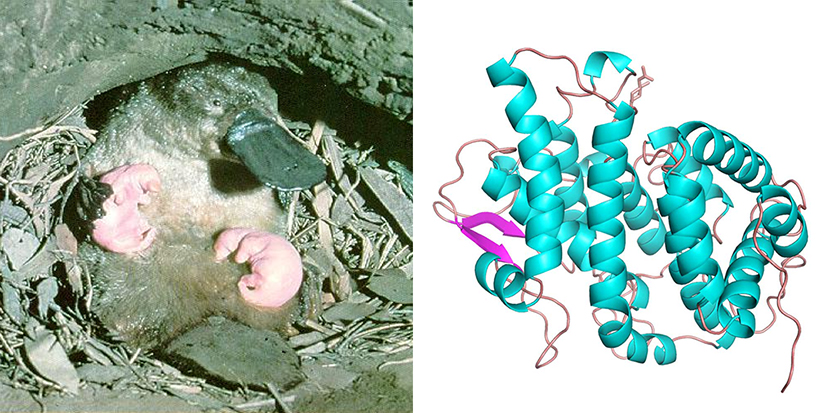
Above left: a platypus mother feeds her young. Above right: The one-of-a-kind ‘Shirley Temple’ protein (monotreme lactation protein) discovered in platypus milk
Scientists at CSIRO replicated the monotreme lactation protein (MLP) using the Australian Synchrotron and CSIRO’s state of the art Collaborative Crystallisation Centre (C3), then deciphered its structure to get a closer look.
What they found was a never-before-seen 3D fold, which they dubbed the ‘Shirley Temple’, in tribute to the former child-actor’s distinctively curly hair.
“This curl structure suggests a new mechanism for killing bacteria and could be a different way of providing antimicrobial protection and overcoming antimicrobial resistance,” Dr Sharp explained.
“Antimicrobial resistance occurs when bacteria, that were once responsive to antimicrobial treatments like antibiotics, build up a resistance and then pass that resistance on to their next generation. This leads to ineffective treatments and more persistent infections.
[testimonial_text]If we treat the bacteria using a different mechanism that’s unrelated to the protein it has developed a resistance to, we can kill it using this new approach. It might then take decades for the bacteria to combat that mechanism and develop new resistance.[/testimonial_text]
[testimonial_picture name=”Dr Julie Sharp” details=”Deakin’s Institute for Frontier Materials (IFM)”]
 [/testimonial_picture]
[/testimonial_picture]Dr Sharp said she originally began researching platypus milk because of the way young platypus are fed. Female platypus don’t have nipples – instead their milk is released through a mammary pad in the skin – and newly-hatched platypus lick the milk from their mother’s stomach, along with all the bacteria living there.
“The reason we started looking at the milk was because platypus young hatch at such an early developmental stage (like a foetus) and the only thing supporting their growth and development is their mother’s milk. As the young platypus is not attached to a placenta, it doesn’t have the internal attachment to its mother like other mammals where she provides developmental signals. The milk alone is responsible for the young’s’ development to the next stage. We wanted to know what properties allow it to do this, and what the potential might be to use those properties to help the development of pre-term babies.”
Dr Sharp and her team collected cells and sequenced the ribonucleic acid (RNA) in a sample of platypus milk, which allowed them to see the profile of the proteins contained in it.
“We discovered a novel protein that didn’t appear to exist anywhere in the literature and, when we tested it, we found it was antimicrobial. It doesn’t appear to be broad spectrum, but is specific to Staphylococcus aureus – which causes golden staph infections – and Enterococcus faecalis, found in faecal matter and the species most predominant in polluted aquatic environments,” she said.
“We think the fact that it only appears in milk from monotremes like platypus might have something to do with protecting the young from bacteria that might be present due to the way they feed from their mother’s skin.”
Dr Sharp said that, having worked out the structure of the protein and what it does, the next step is to work out how.
“Once we know how, and if the novel fold has something to do with it, we can then create peptides that contain only the fold structure or artificially-engineered mimics. That could actually end up strengthening its ability to kill bacteria,” she explained.
Watch the “Platypus with Superpowers” story here. Reproduced with permission from Totally Wild.
Published by Deakin Research on 14 June 2018



