A cancer-free future is the end goal, improved tumour detection is the life-changing stepping stone.
Now, how can this future be created?
It starts with equipping medical professionals with the framework to locate tumours before they spread. Gathering more information about neuroendocrine tumours, in particular, is key.
When tumour meets detection
Neuroendocrine tumours (NETs), the Director for the Centre for Cellular and Molecular Biology Alfred Deakin Professor Leigh Ackland explains, can occur in a wide range of systems in the body including organs that are critical components of our digestive system.
From the pancreas to the small and large intestine, these tumours have far-reaching hands.
This is where the difficult nature of these tumours reveals itself: how can these tumours be better detected when their behaviour is so varied?

Researchers note this has contributed to NETs patients often reporting with very general symptoms or not reporting until the cancer is very advanced.
Even the site of origin can be challenging to pinpoint, when tumours have moved away from where they started. Putting your finger on the ‘warning signs’ is integral to patients wellbeing and quality of life.
Frequently, cancers provide ‘clues’ as to its type and best treatment. Although, this isn’t the case for many neuroendocrine tumours.
Instead, it requires oncologists and medical researchers to put on their detective hats, to find the characteristics expressed in the cancer cells before they go undetected.
“We are trying to relate the characteristics of the cells and find out how they differ between patients at different stages of the disease. Also why some tumours are more fatal than others.
“That sort of work hasn’t been done before,” says Professor Ackland.
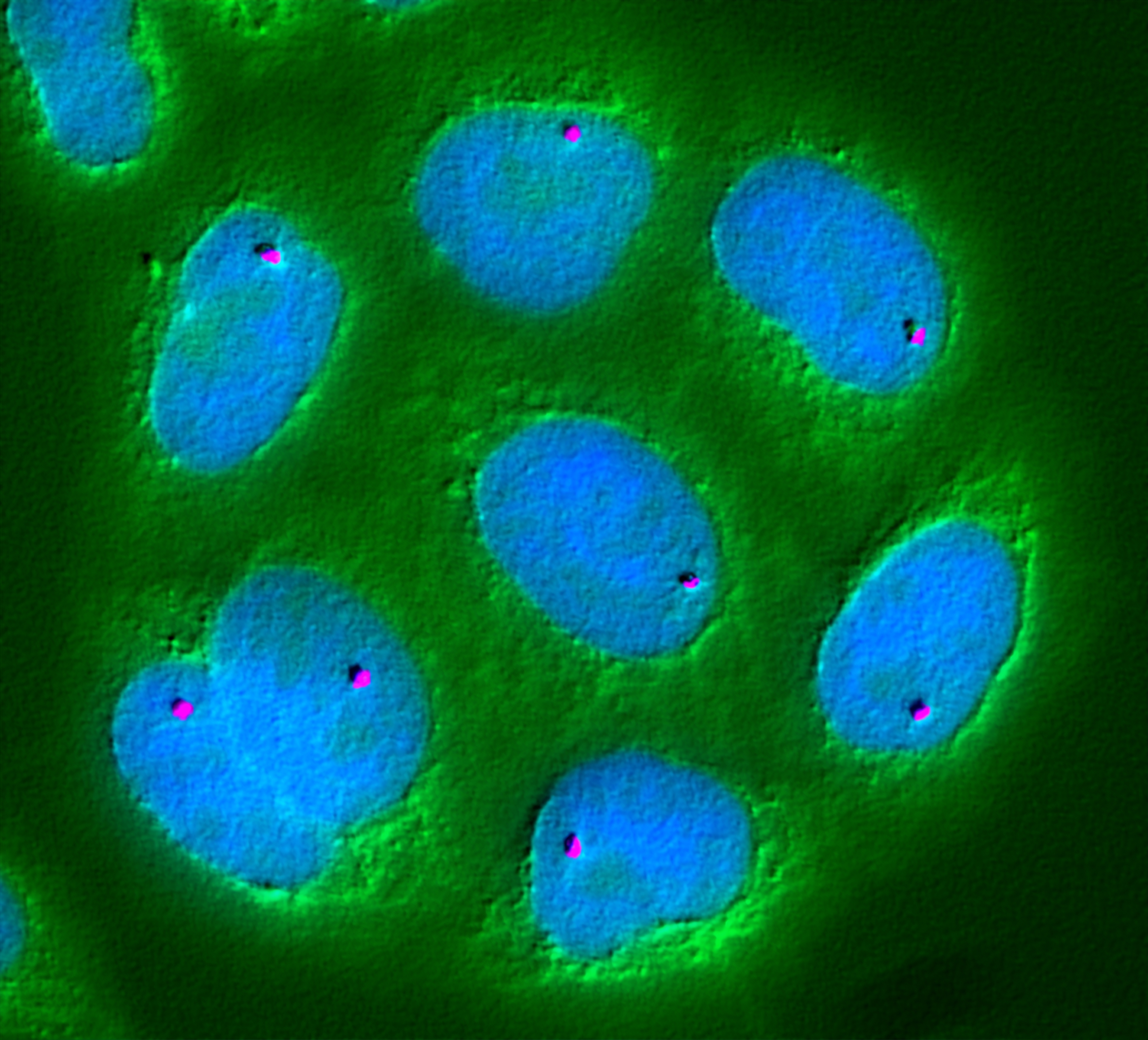
It starts with spotting the differences between cancer cells and normal cells. To notice the various proteins being expressed. To understand what makes these tumours unique.
While this research is in its initial stages, researchers hope to “develop a molecular fingerprint that will describe the particular tumour and provide information to the oncologist about potentially what the best treatment is and at what stage the disease is.”
Rethinking treatment methods
Traditionally, cancer treatments have been designed to kill all dividing cells. Cancer cells included.
These dividing cells are similar to the job of a photocopier; to make a copy of an original to generate something new.
While these treatments can be life-saving for patients, they also have the ability to attack normal, healthy cells. Professor Ackland refers to these methods as sled-hammer treatments because they kill all cells which are going through this copying process.
“The fact is,” she says, “that the cancer cells are dividing, but lots of other cells in the body are dividing because that is part of their normal function – like your gut cells and your skin cells.”
When attacking cells that are still healthy, many side-effects can occur. In areas like the gut, skin, hair and other systems patients can showcase the weight of this disease on their bodies.
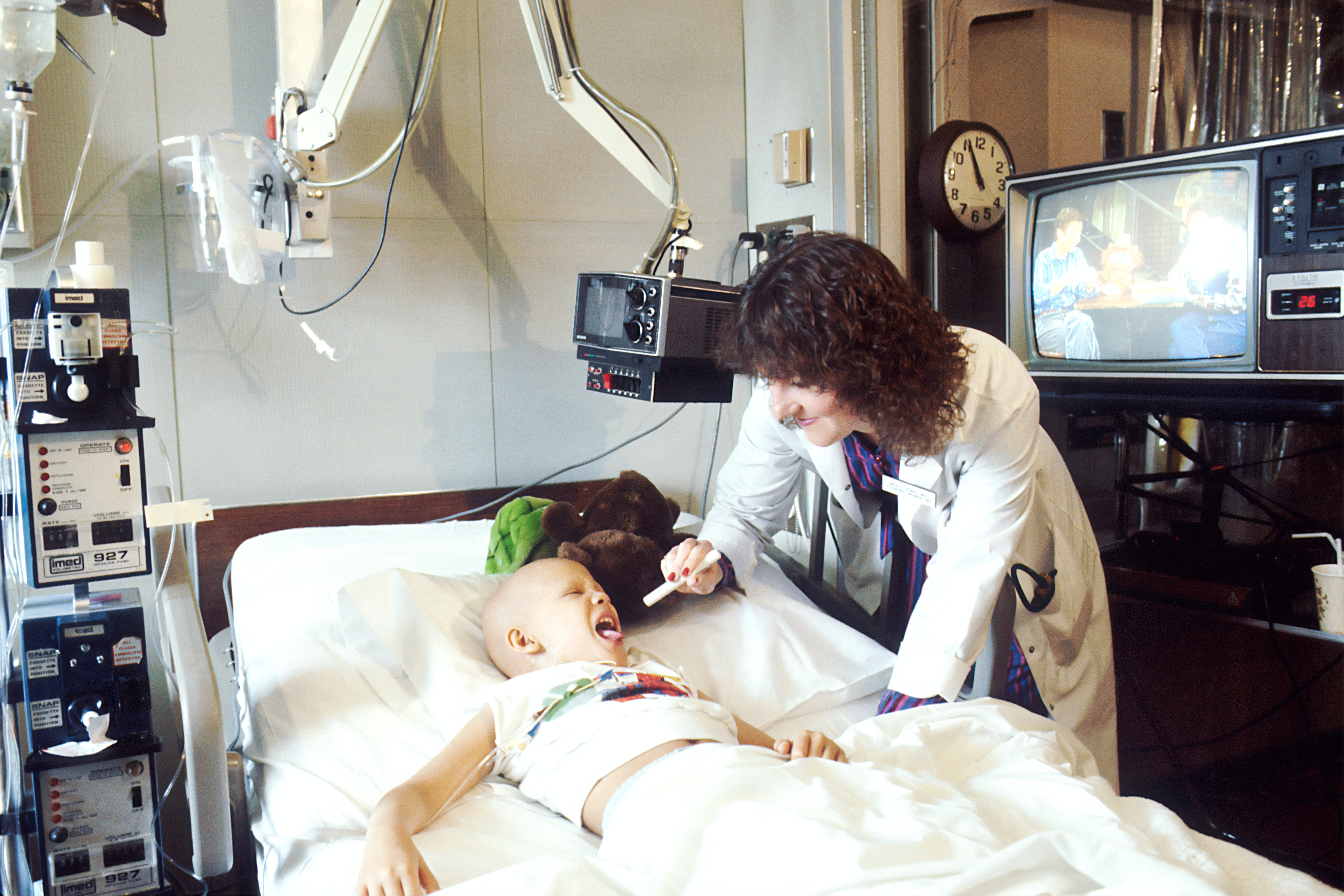
For instance, many patients experience hair loss because of their treatment, because their hair cells are also being attacked.
“So, the idea behind our study is that we can identify the individual characteristics of each cancer and then use those characteristics to target treatments that only affect the cancer cells,” says Professor Ackland.
The potential for the future of this research could greatly benefit patients undergoing these targeted treatments, without fear of other healthy systems becoming corrupted.
Beyond the tissue
Typically, when cancer spreads within the tissue it can also travel into the bloodstream to go to a new site; here, a secondary tumour can form.
Professor Ackland notes that more research needs to be done in this area, because, a cancer cell has to fundamentally change its characteristics in order to enter into the blood.
“People’s bodies create tumour cells fairly frequently but they get eliminated by the immune systems”.
“But in cancers, while there may not be an obvious immune defect, they are not getting destroyed by the immune system.
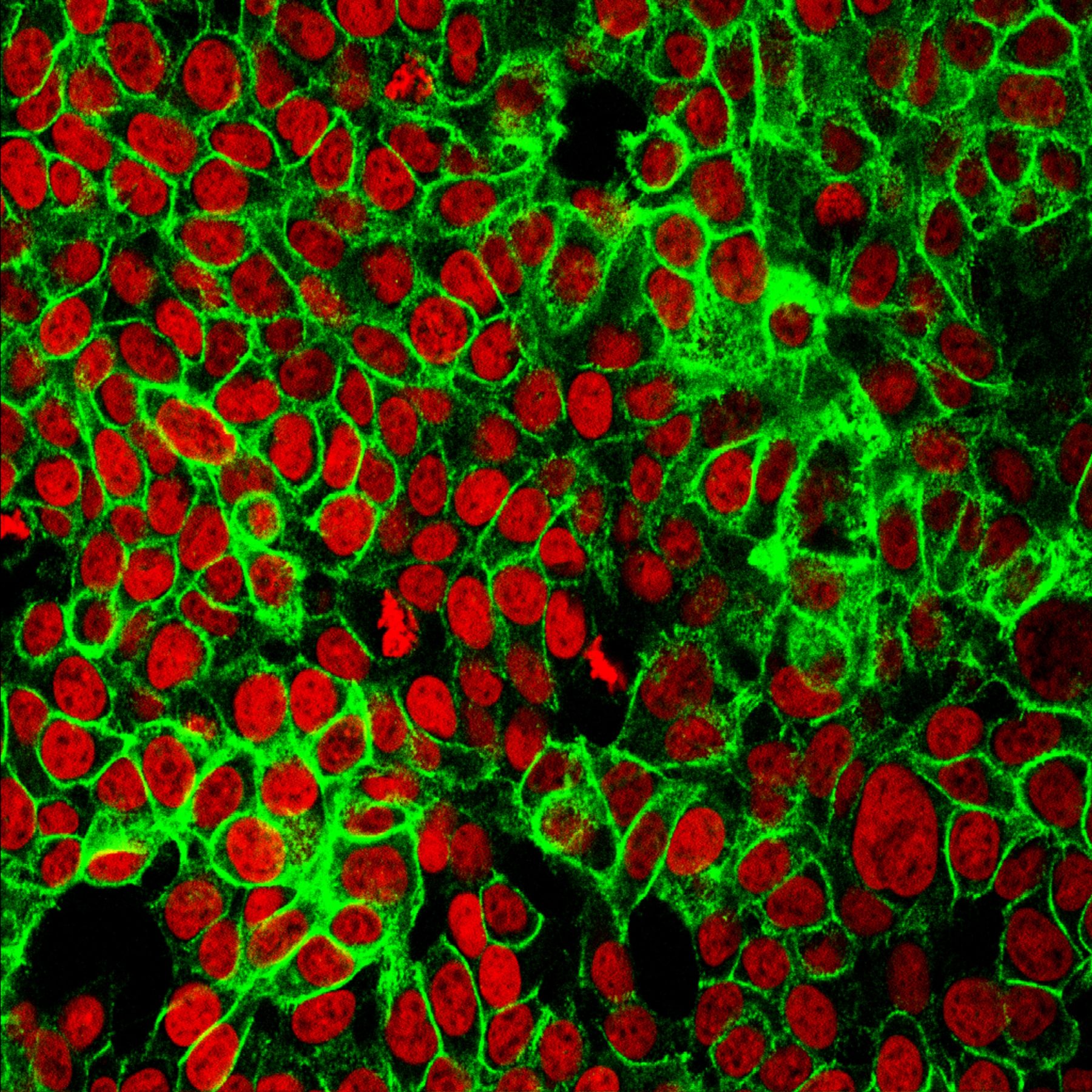
“So, they [the tumours] can persist in the blood and then they can squeeze through the lining of the blood vessels to go to a new site.”
The formation of secondary tumours can significantly worsen the health of patients.
By extracting tissue samples from patients, researchers hope to gain a deeper understanding of the origins of neuroendocrine tumours and prevent the formation of secondary tumours with early intervention.
A philanthropic approach
Perhaps, the challenge is then bolstering the conversation around these lesser-known tumours to fund this research and highlight the need for dynamic solutions to overcome this disease.
Professor Ackland champions the support of philanthropic organisations, like that of the Bourne Foundation in aiding and driving research.
She was recently awarded $300,000 from the Bourne Foundation for cancer research over 3 years. With the backing of people who have experienced cancer, the goal towards ending this disease becomes easier to reach.
From grass-roots people power to the lab, the lens of knowledge can widen a little further.
“It’s fantastic that people who are affected by this have actually decided to commit to helping get rid of this disease and it would be great to extend into other aspects of disease, not just cancers,” says Professor Ackland.
“People are showing they have the foresight to initiate these research projects if they want, they are not just waiting for a higher power to do something – that applies not just to medicine but to social issues as well.”